
Dangerous Disinformation: Using health discussion networks to mitigate damaging effects on PrEP uptake from frivolous advertising of Tenofovir disoproxil fumarate (TDF) lawsuits on social media
Drew A. Westmoreland, Ian W. Holloway, Demetre Daskalakis, Christian Grov
Background: Tenofovir disoproxil fumarate/Emtricitabine (TDF/FTC, Truvada) as pre-exposure prophylaxis (PrEP), an effective method to prevent HIV, was FDA approved in 2012, but PrEP uptake among key populations has been slow in the U.S. In October 2019, Tenofovir Alafenamide/Emtricitabine (TAF/FTC, Descovy) was approved by the FDA and marketed as “safer” and “more effective” than TDF/TFC. Subsequently, ads have emerged on social media to recruit plaintiffs for lawsuits regarding non-demonstrated harms caused by TDF/FTC. The purpose of this study is to identify the role that discussion networks may have in mitigating effects of these negative ads on PrEP uptake and continuation.
Methods: In the Together 5,000 (T5K) cohort, we are monitoring the reach and impact of these negative ads on participants’ attitudes toward PrEP. T5K is a U.S. nationwide study of HIV-vulnerable men and transgender persons who have sex with men to identify missed opportunities for HIV prevention. Enrollment occurred from October 2017 to June 2018. In October 2019, we began 24-month assessments which collect data on participants’ discussion networks (e.g., PrEP discussions) and the reach and impact of TDF lawsuit ads. This abstract presents results from responses received as of January 2020 (n = 1,175), but full cohort data (n = 5,000) will be available in May 2020.
Results: Over half (60%) reported that they had seen TDF-related lawsuit ads on social media. Twenty-six (26.3%) percent reported they would probably or definitely not start PrEP and 23.6% reported they would probably or definitely not stay on PrEP after seeing these ads. Over one-third (38.1%) reported that they (strongly) agreed that these ads made them think TDF/FTC as PrEP was not safe. Twenty-three percent (23.2%) knew no one on PrEP, down slightly from 27.4% at baseline. Unadjusted multilevel logistic analyses indicate that knowing more people on PrEP reduced negative impacts of lawsuit ads on starting and continuing on PrEP. Many participants reported that they would discuss PrEP with a main partner (45.2%) and a LGBTQ-identified close friend (54.1%). Only thirty-six (35.9) percent reported that they would discuss PrEP with a medical provider, and, in unadjusted analyses, participants who would not talk to a medical provider had a higher odds of being negatively impacted by the ads. Preliminary analyses also indicate that being willing to discuss PrEP with family, friends, and sex partners reduced the negative influence of these ads on PrEP decision-making. For example, participants who would discuss PrEP with immediate family (OR = 2.1, 95% CI: 1.4-3.0), a main partner (OR = 1.7, 95% CI: 1.3-2.4) or an LGBTQ-identified close friend (OR = 2.0, 95% CI: 1.5-2.7) had a higher odds of probably or definitely starting PrEP despite the lawsuit ads.
Conclusions: T5K participants’ PrEP attitudes were negatively impacted by TDF-related lawsuit ads on social media. As the patent expires and more affordable versions of TDF/FTC are developed, it will likely become the feasible alternative for large-scale interventions. Leveraging PrEP candidates’ and users’ own social support networks may be an effective way to disseminate messages to counter these negative lawsuit ads. 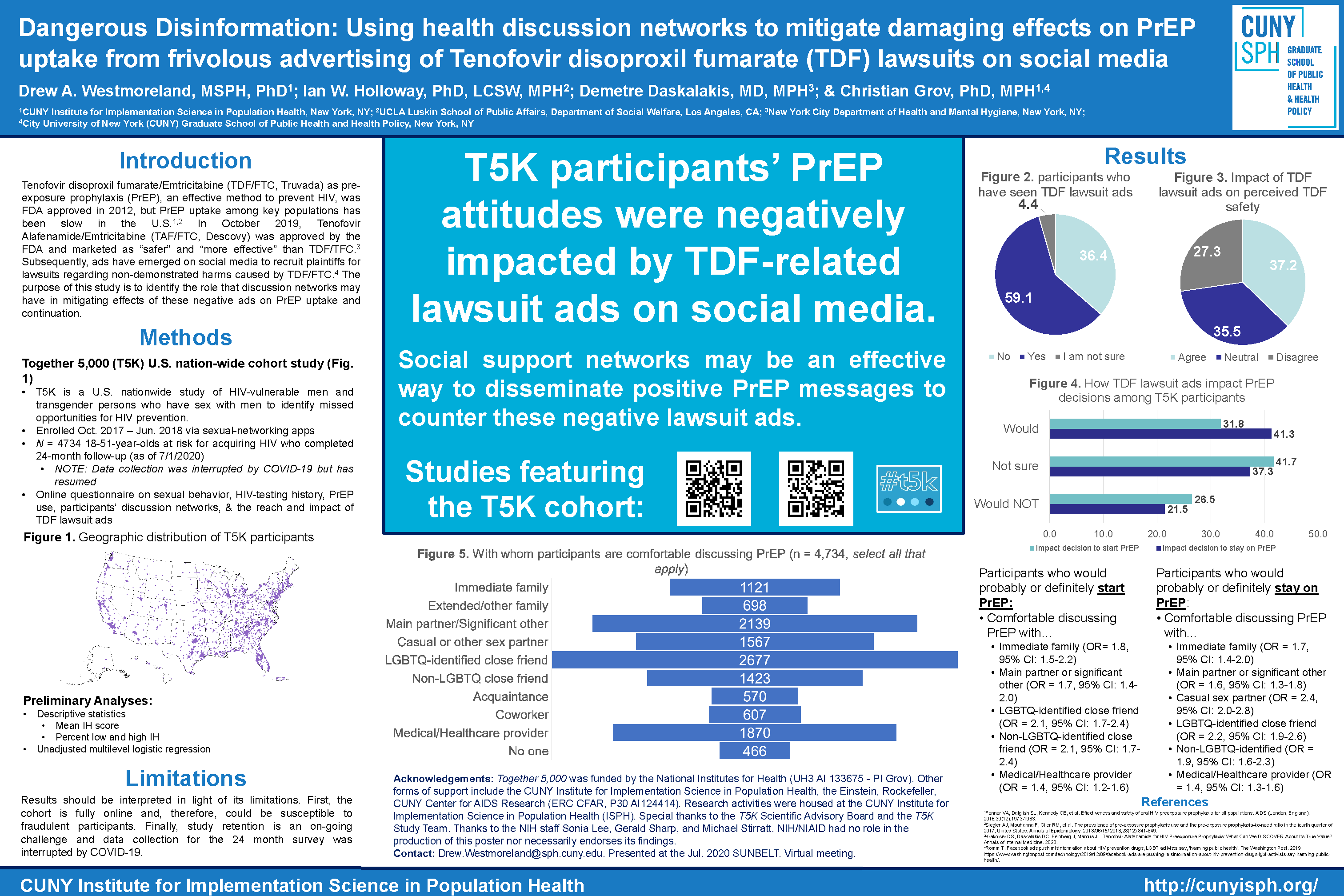
← Schedule

